Unusual Transition Metal Oxo Complexes
Learn more about our investigations of late transition metal oxo reactivity and the influence of electric fields here.
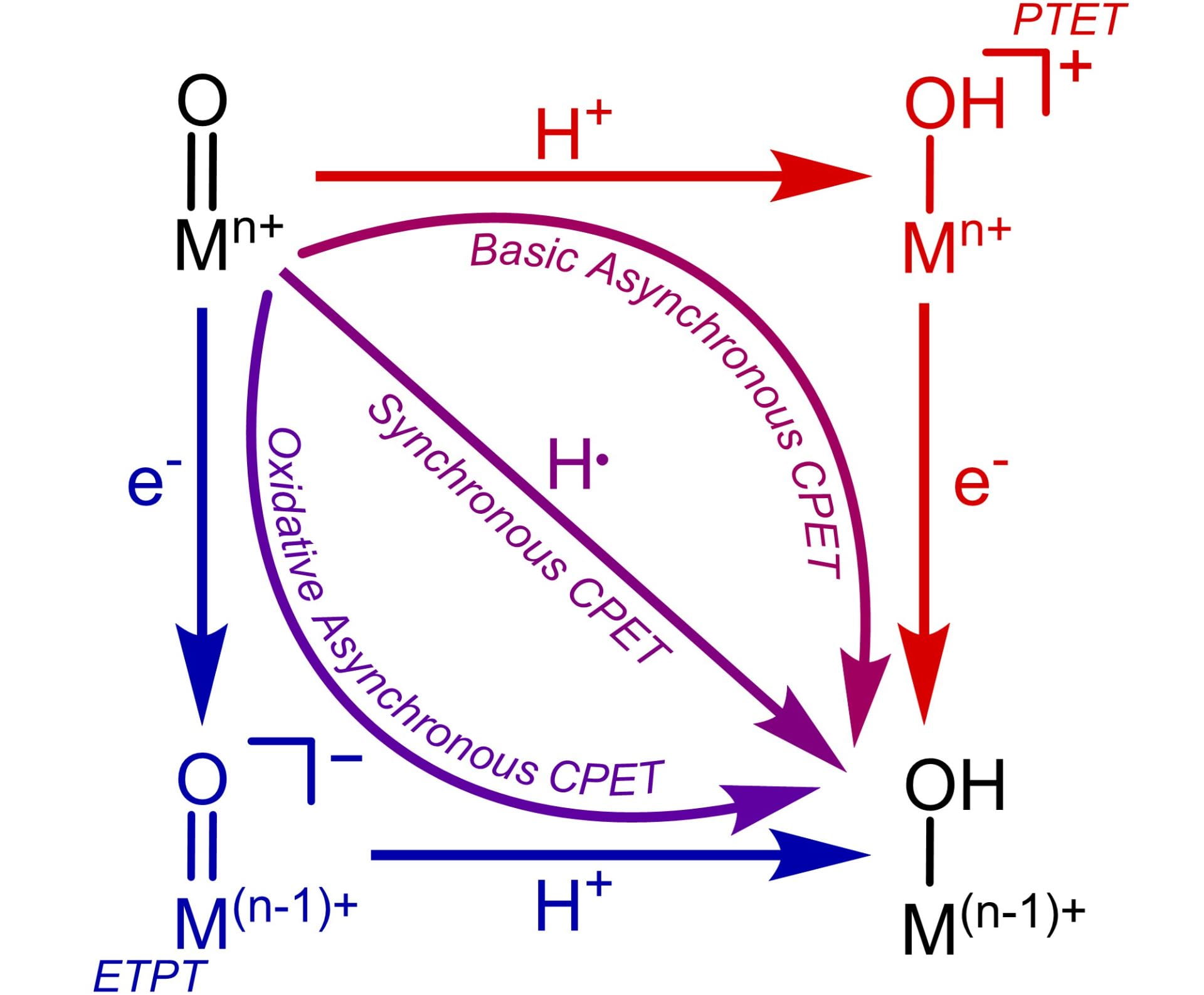
C-H Activation in Late Metal Oxos
The activation of C-H bonds is central to a variety of reactions across biology and synthetic chemistry. In many instances, such as the degradation of pharmaceuticals by cytochrome P450, this C-H activation is mediated by a transition metal oxo complex. Years of research have established that in most cases, metal oxos are most reactive with the weakest C-H bonds, operating via a synchronous CPET mechanism. However, we have recently shown that in the case of a Co(III)-oxo complex, reactivity occurs via a basic asynchronous CPET mechansim, resulting in the oxo complex being most reactive with the most acidic C-H bonds.
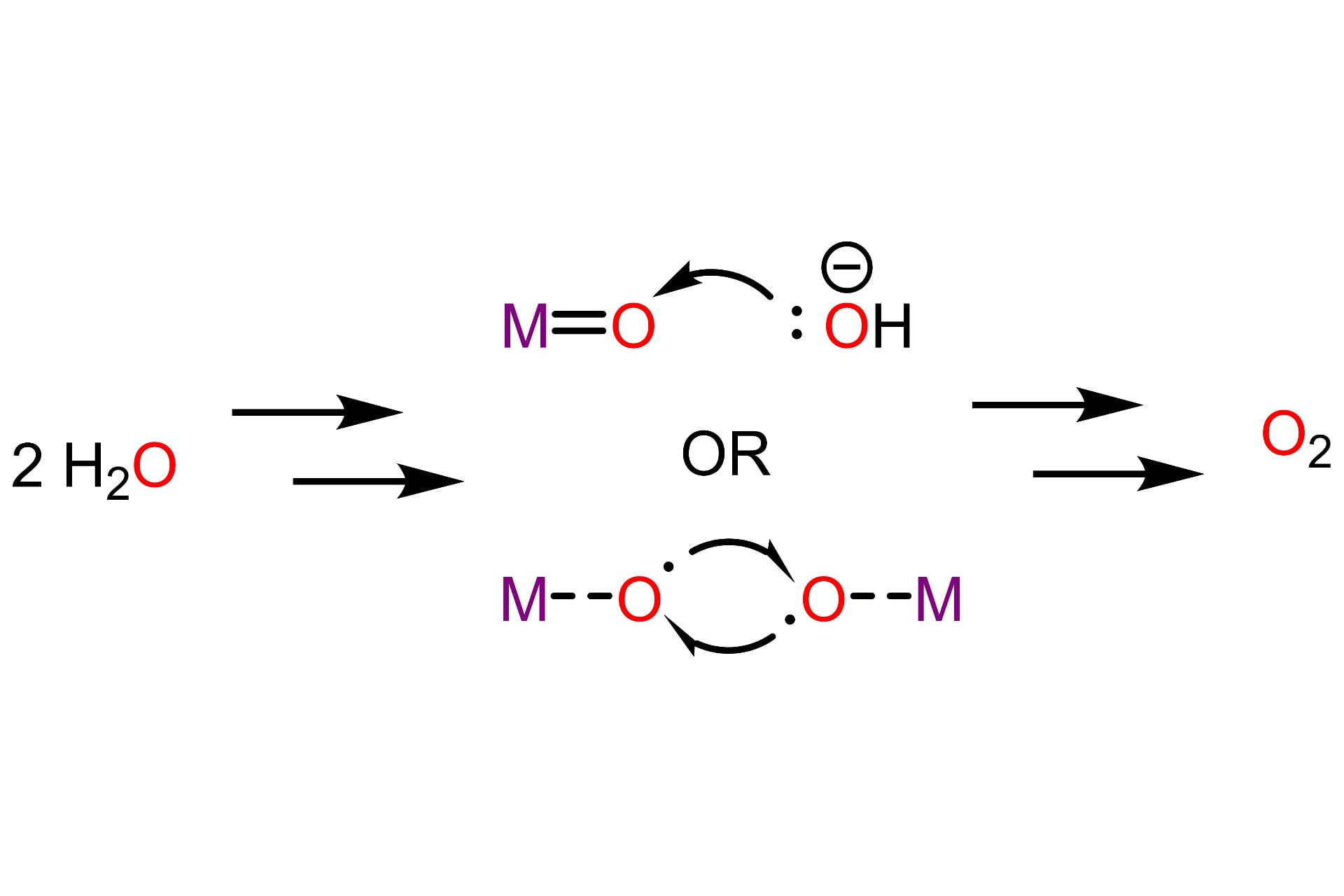
Mechanisms of O-O Bond Formation
Oxygen evolution is central to many alternative energy schemes that have been inspired by water oxidation carried out during photosynthesis. However, a general understanding of the mechanism by which this reaction occurs is still lacking. There are two main proposals for the mechanism of O-O bond formation: nucleophilic attack of an external hydroxide or water ligand, or radical coupling between two metal oxo units. Recently, there has been much interest in the idea of oxo-oxo coupling. We seek to observe either of these two mechanisms in our systems, and expect that the unusual electronic structures obtained from distal charges or psuedo-tetrahedral geometries will favor a radical coupling mechanism.
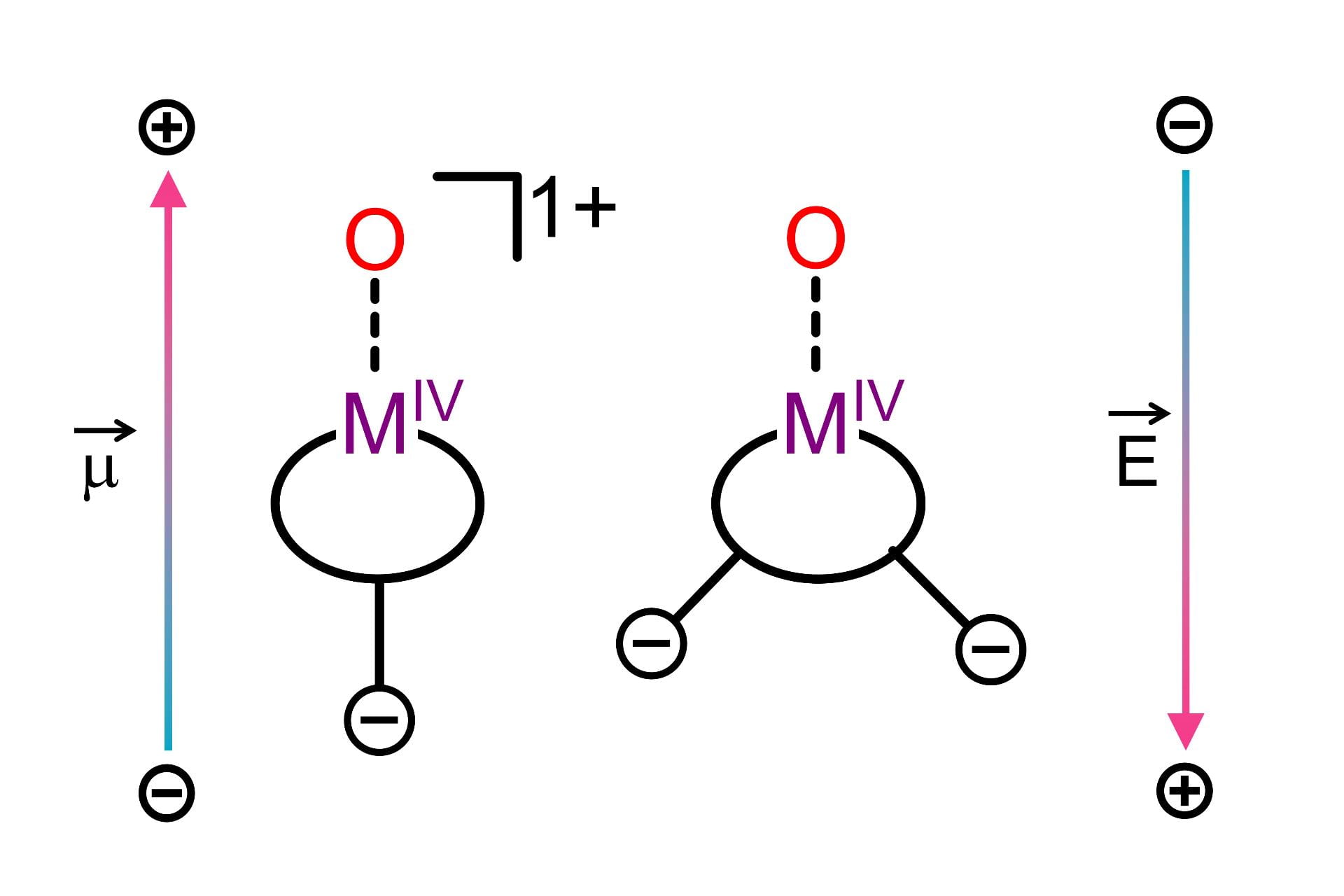
Influence of Electric Fields
Electric fields induce the polarization of electron density within a molecule, and have been cited as contributing to the enhanced catalytic activity observed in enzymes. We are studying the effects of electric fields on the electronic structure and reactivity of metal oxo complexes by designing ligand scaffolds with charged groups distal to the metal center. These charged groups will lead to an electric field gradient across the molecule and allow us to intentionally and precisely vary the magnitude and direction of the field by placing the charges at different locations.
Selected publications:
“Nickel(II)-Methyl Complexes Adopting Idealized Seesaw Geometries” *Hill, E.A.; *Zhao, N.; Filatov, A. S.; Anderson, J. S. Chem. Commun. 2020, Advance Article. Read it!
“Experimental Evidence for pKa-Driven Asynchronicity in C-H Activation by a Terminal Co(III)-Oxo Complex” Goetz, M. K.; Anderson, J. S. J. Am. Chem. Soc. 2019, 141, 4051-4062. Read it!
“Imidazole for Pyridine Substitution Leads to Enhanced Activity Under Milder Conditions in Co Water Oxidation Electrocatalysis” *McMillion, N. D.; *Wilson, A. W.; Goetz, M. K.; Chang, M-C.; Lin, C-C.; Feng, W-J.; McCrory, C. C.; Anderson, J. S. Inorg. Chem. 2019, 58, 1391-1397. Read it!
“Isolation of a Terminal Co(III)-Oxo Complex” Goetz, M. K.; Hill, E. A.; Filatov, A. S.; Anderson, J. S. J. Am. Chem. Soc., 2018, 140, 13176-13180. Read it!
“Isolable Iodosylarene and Iodoxyarene Adducts of Co and Their O-atom Transfer and C-H Activation Reactivity” Hill, E. A.; Kelty, M. L.; Filatov, A. S.; Anderson, J. S. Chem. Sci., 2018, 9, 4493-4499. Read it!
