Metal-Organic Materials
Learn more about our approaches to novel redox-active, magnetic, and porous materials here.
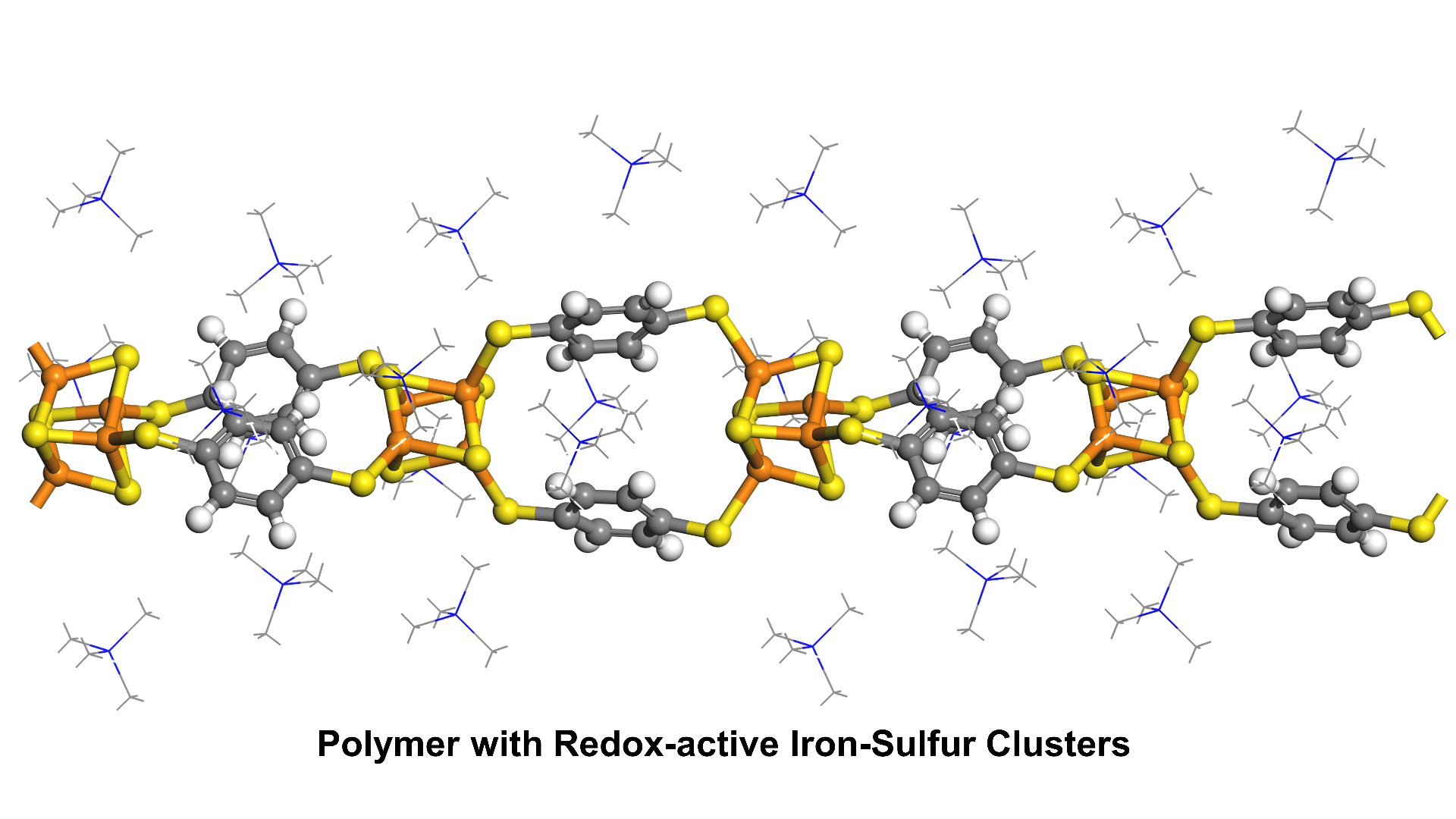
Sulfur-based linkers
Better energetic matching between nodes and linkers should increase electrical conductivity and magnetic coupling in the resulting materials. Sulfur-based linkers have a better energetic match to transition metal nodes than more common oxygen- or nitrogen-based ligands. We are developing new synthetic strategies to simplify the use of highly reactive S-based linkers in making new conductive materials.
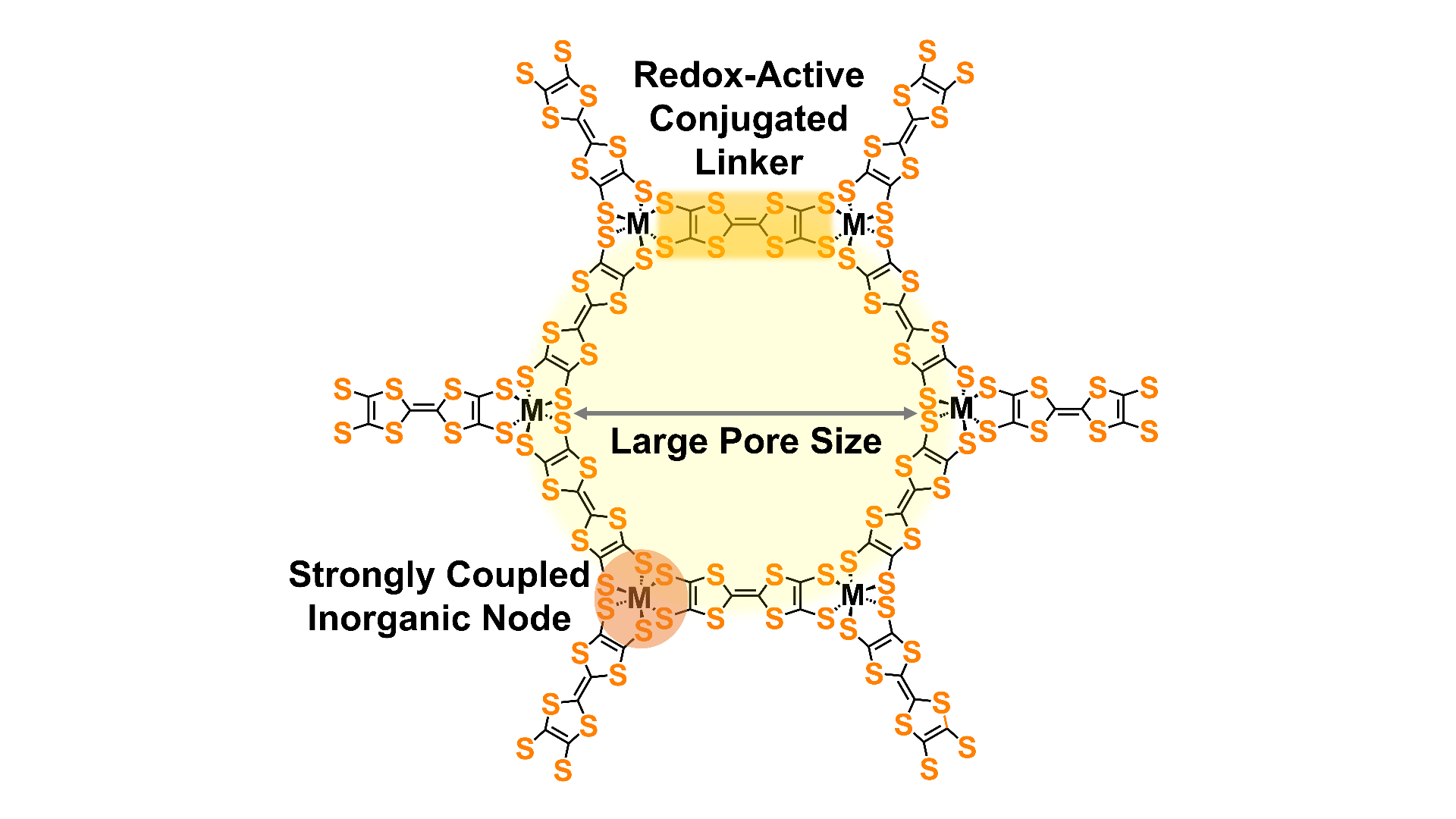
Redox-active nodes and linkers
Redox reactions on linkers and nodes allow materials to store electrical charge in batteries and supercapacitors. The use of redox-active nodes and linkers also allows facile redox doping of materials to improve electrical conductivity. We are pursuing new redox-active linkers and also exploring the use of redox-active metal clusters as nodes.

Installation of radical linkers
By inserting an S = 1/2 linker, direct exchange between paramagnetic metal nodes can be realized via coupling to the bridging radical ligand, allowing for long-distance magnetic ordering. Simultaneously, fusion of open-shell linkers and metal nodes gives rise to systems with delocalized charge carriers. Studying the synergistic effects of magnetism and charge transport could pave the way to next generation spintronics devices.
Selected publications:
“Catalytic, Spectroscopic, and Theoretical Studies of Fe4S4-Based Coordination Polymers as Heterogenous Coupled Proton–Electron Transfer Mediators for Electrocatalysis” Jiang, N.; Darù, A.; Kunstelj, Š.; Vitillo, J.; Czaikowski, M.; Filatov, A. S.; Wuttig, A.; Gagliardi, L.; Anderson, J. S. J. Am. Chem. Soc. 2024, 146, 17, 12243–12252. Read it!
“Aliovalent Substitution Tunes Physical Properties in a Conductive Bis(dithiolene) Two-Dimensional Metal−Organic Framework” Wang, L.; Darù, A.; Jangid, B.; Chen, J-H.; Jiang, N.; Patel, S. N.; Gagliardi, L.; Anderson, J. S. J. Am. Chem. Soc. 2024, 146, 17, 12063–12073. Read it!
“Redox Chemistry Mediated Control of Morphology and Properties in Electrically Conductive Coordination Polymers: Opportunities and Challenges” Wang, L.; Anderson, J. S. Chem. Mater. 2024, 36, 9, 3999–4010. Read it!
“Cluster-Spin-Glass Magnetic Behavior and Morphology in the Coordination Polymer Alloys FeyCo1-yBTT” Ritchhart, A.; Chen, Z.; Behera, A.; Jeon, l-R.; Chapman, K. W.; Vaikuntanathan, S-r.; Anderson, J. S. J. Am. Chem. Soc. 2023, 145, 44, 24089–24097. Read it!
“Potential for exciton condensation in a highly conductive amorphous polymer” Schouten, A. O.; Klevens, J. E.; Sager-Smith, L. M.; Xie, J.; Anderson, J. S.; Mazziotti, D. A. Phys. Rev. Materials, 2023, 7, 045001. Read it!
“Broad Electronic Modulation of 2D Metal-Organic Frameworks Over Four Distinct Redox States” Wang, L.; Sarkar, A.; Grocke, G. L.; Laorenza, D. W.; Cheng, B.; Ritchhart, A.; Filatov, A. S.; Patel, S. N.; Gagliardi, L.; Anderson, J.S. J. Am. Chem. Soc. 2023, 145, 15, 8486–8497. Read it!
“The Structure and Magnetic Properties of a New Family of Pseudo-1D Chromium Thiolate Coordination Polymers” Ritchhart, A.; Filatov, A. S.; Jeon, I-R.; Anderson, J. S. Inorg. Chem. 2023, 62, 6, 2817–2825. Read it!
“Pre-Synthetic Redox Gated Metal-to-Insulator Transition and Photothermoelec-tric Properties in Nickel Tetrathiafulvalene-Tetrathiolate Coordination Polymers” *Xie, J.; *Pan, J.-A.; Cheng, B.; Ma, T.; Filatov, A. S.; Patel, S. N.; Park, J.; Talapin, D. V.; Anderson, J. S. J. Am. Chem. Soc. 2022, 144, 41, 19026-19037. Read it!
“Linker Redox Mediated Control of Morphology and Properties in Semiconducting Iron-Semiquinoid Coordination Polymers” Wang, L.; Papoular, R. J.; Horwitz, N. E.; Xie, J.; Sarkar, A.; Campisi, D.; Zhao, N.; Cheng, B.; Grocke, G. L.; Ma, T.; Filatov, A. S.; Gagliardi, L.; Anderson, J. S. Angew. Chem. Int. Ed. 2022, 61, e20220784. Read it!
“An Intrinsically Glassy Metallic Coordination Polymer Showing Thermally and Aerobically Robust Conductivity” Xie, J.; Ewing, S.; Boyn, J-N.; Cheng, B.; Filatov, A. S.; Ma, T.; Zhao, N.; Itani, R.; Sun, X.; Cho, H.; Patel, S. N.; Talapin, D. V.; Park, J.; Mazziotti, D. A.; Anderson, J. S. Nature 2022, 1-6. Read it!
“Donor-Acceptor Conjugated Copolymers Containing Transition-Metal Complex: Intrachain Magnetic Exchange Interactions and Magneto-Optical Activity” *Liu, X.; *Xie, J.; Niklas, J.; Turner, E. E.; Yuan, D.; Anderson, J. S.; Rack, J. J.; Poluektov, O. G.; Yu, L. Chem. Mater. 2022, 34, 5740-5747. Read it!
“Steric and Electronic Effects of Ligand Substitution on Redox-Active Fe4S4-Based Coordination Polymers” *Salinas, O.; *Xie, J.; Papoular, R. J.; Horwitz, N. E.; Elkaim, E.; Filatov, A. S. Anderson, J. S. Dalton Trans., 2021, 50, 10798-10805. Read it!
“Magnetically Coupled Iron Azide Chains” Lugosan, A.; Kawamura, A.; Dickie, D. A.; Zeller, M.; Anderson, J. S.; Lee, W-T. Inorganica Chim. Acta, 2021, 516, 120150. Read it!
“Heavy Chalcogenide-Transition Metal Clusters as Coordination Polymer Nodes” *Xie, J.; *Wang, L.; Anderson, J. S. Chem. Sci. 2020, 11, 8350-8372. Read it!
“Synthesis, Modular Composition, and Electrochemical Properties of Lamellar Iron Sulfides” Horwitz, N. E.; Shevchenko, E. V.; Park, J.; Lee, E.; Xie, J.; Filatov, A. S.; Anderson, J. S. J. Mat. Chem. A 2020, Advance Article. Read it!
“Sulfonate-Ligated Coordination Polymers Incorporating Paramagnetic Transition Metals” Kawamura, A.; Filatov, A. S.; Anderson, J. S. Eur. J. Inorg. Chem. 2019, 21, 2613-2617. Read it!
“Slow Magnetic Relaxation of Co(II) Single-Chains Embedded within Metal-Organic Superstructures” Kawamura, A.; Filatov, A. S.; Anderson, J. S.; Jeon, I-R. Inorg. Chem. 2019, 58, 3764-3773. Read it!
“Redox-Active 1D Coordination Polymers of Iron-Sulfur Clusters” *Horwitz, N. E.; *Xie, J.; Filatov, A. S.; Papoular, R. J.; Shepard, W. E.; Grahn, M. P.; Gilder, C.; Anderson, J. S. J. Am. Chem. Soc. 2019, 141, 3940-3951. Read it!
“Incorporation of Pyrazine and Bipyridine Linkers with High-Spin Fe(II) and Co(II) in a Metal-Organic Framework” Kawamura, A.; Greenwood, A. R.; Filatov, A. S.; Gallagher, A. T.; Galli, G.; Anderson J. S. Inorg. Chem. 2017, 56, 3349-3356. Read it!
